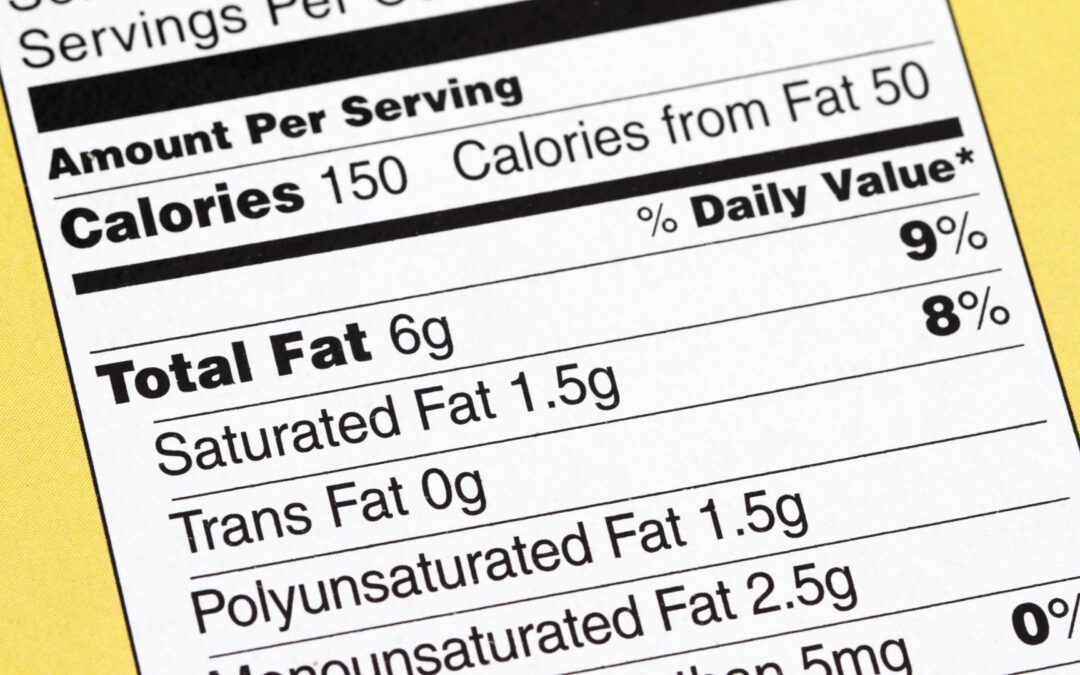
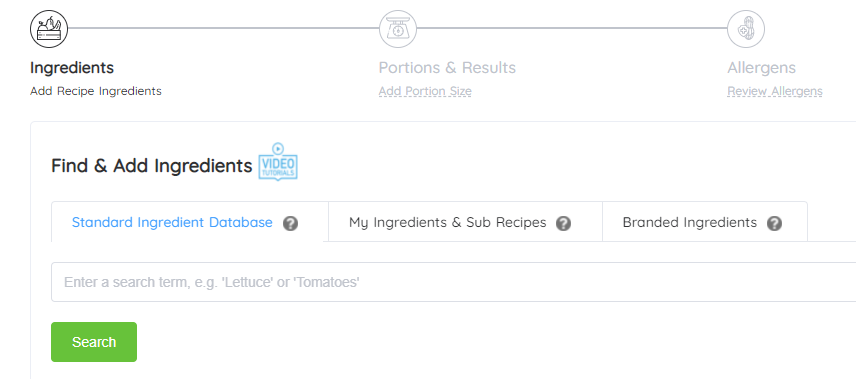
Florham Park, NJ, February 2024 – LabelCalc, a leading provider of online nutrition analysis software, is thrilled to announce a rebranding initiative that includes a new corporate brand identity, redesigned logo and website. The rebranding reflects LabelCalc’s...