OverDate: Wednesday, November 20th | 11 AM ET
Complimentary Webinar
There’s now less than a year to prepare for the new food traceability requirements coming from the U.S. Food and Drug Administration (FDA) in January 2026. Although food traceability has been a requirement since the introduction of The Bioterrorism Act of 2002, the impending changes invariably bring with them plenty of uncertainty and confusion.
A recent webinar hosted by Food Dive, titled Mastering the FDA’s Food Traceability Rules for Food Processors with Data, aimed to offer a little clarity on how to interpret the new requirements.
Featuring Tracy Herb, Product Manager at LabelCalc and Teresa Finn, Product Manager for Food and Beverage at Datacor, the session explored the nuances of the new rules and highlighted the potential pitfalls for food manufacturers.
Room for Improvement
Section 204(d) of the Food Safety Modernization Act (FSMA) aims to identify and track our food sources more effectively.
The Food Traceability Proposed Rule calls for the FDA to establish the Food Traceability List – identifying foods that are considered “higher risk” – and put in place strict record-keeping requirements to keep consumers safe and healthy.

The supply chain is used to the one forward, one back rule, which ensures the FDA can quickly track down the source of any issues, said Herb. It applies to everyone from the farmers and packers through to the distributors and beyond. Unfortunately, not everyone has been implementing it correctly – meaning the average time it takes to identify sources currently stands at 35 days!
As a result, the FDA has been exploring ways to improve the process.
Statistics show that 3.6 million people fall ill each year, with many of these cases resulting in hospitalization, or worse. On the day of the webinar recording, Herb noted there were 14 active outbreaks being monitored by the FDA. Shockingly, the source of these incidents had only been identified for two of these cases.
“In this day and age with so much technology, there really needs to be a way to get this information faster because the food manufacturers do not want to hurt people,” said Herb. “They work so hard to make everything safe and wholesome. It will be to everybody’s benefit to get these solved in a timely manner.”
Preparing for Change
The incoming requirements, though welcome, are both confusing and challenging.
With the clock ticking until their introduction, it’s therefore vitally important that food processors are properly prepared for the changes.
Finn acknowledged the FDA has made a huge amount of resources available to help the industry comply, including detailed videos and contacts should companies have any specific queries.
But to be ready for the incoming changes, manufacturers should also ask themselves the following:
- Do you buy or sell any of the foods that appear on the food traceability list?
- Who provides the raw materials (manufacturer, distributor, retailer)?
- Where do you fall in the supply chain and what is your role? This will help you understand which KDEs apply to you.
- How do you intend to manage the Key Data Elements (KDEs) you’re required to capture, pass forward, and keep?
“I think that there’s going to be some unique challenges with distributors and they’re going to have to really focus on this regulation,” noted Finn.
The webinar highlighted just how important it will be to properly understand the relevance of Traceability Lot Codes. Traceability Lot Codes (TLCs) are provided at certain stages of the food manufacturing process, either during packing or in a transformation process, and are notably different from conventional Lot Codes.
“A Traceability Lot Code cannot be changed, must be kept as a record, must be passed on exactly intact as it’s generated,” noted Herb.
TLCs are generated during “transformation” – which the FDA defines as an event in a food’s supply chain that involves manufacturing, processing, or changing of a food (commingling, repacking, relabeling), or its packaging or packing if a food item is on the FTL. Of note here is that if a distributor is selling a FTL item that is not repacked or relabeled, they must retain the original TLC from their previous supplier. However, if they do repackage or relabel, then they must generate a new TLC.
“That’s kind of those devilish details that we need to to hone in on,” said Finn.
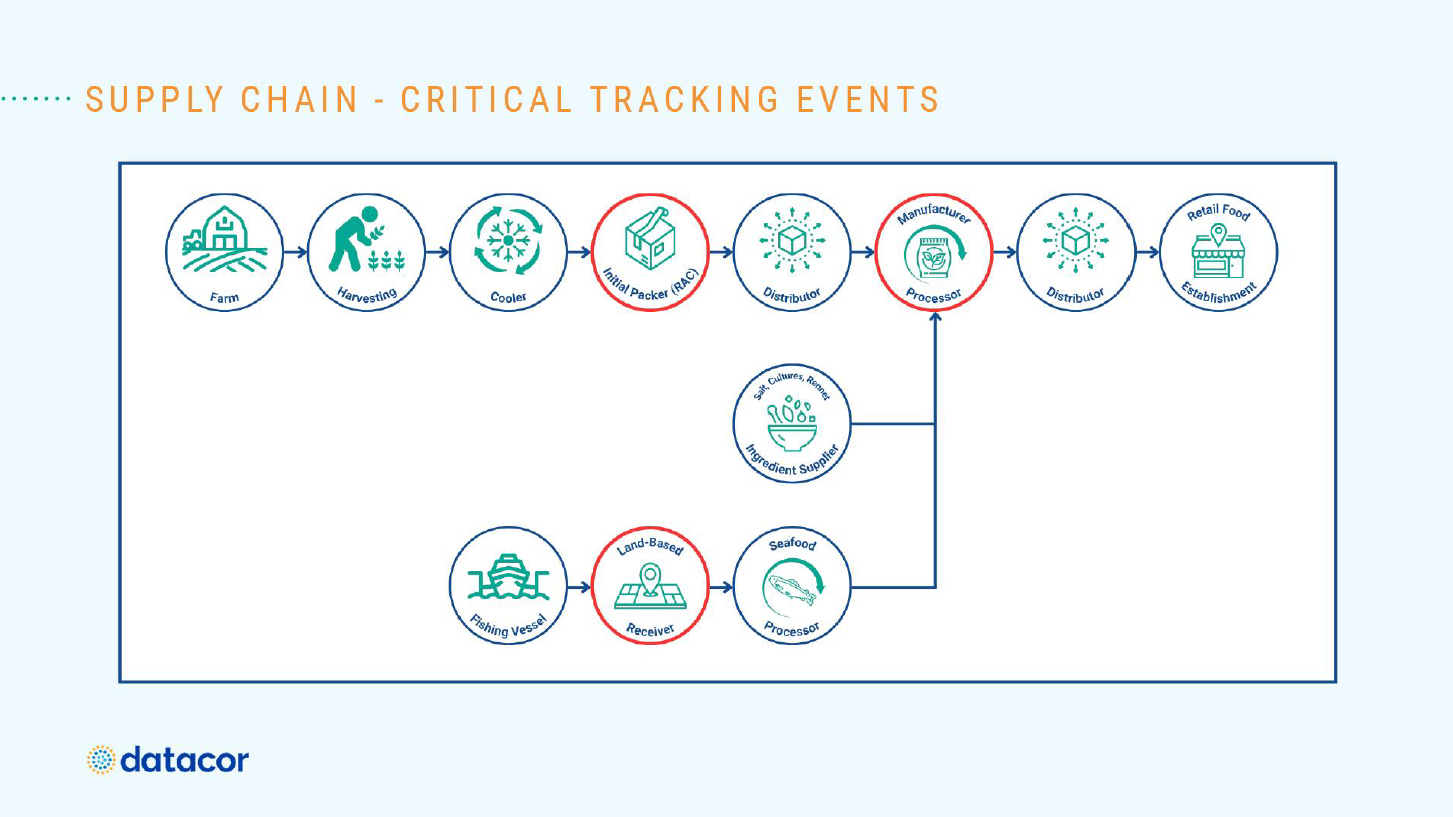
Developing Data-Driven Strategies
Food manufacturers are going to be required to collect and manage considerable amounts of data – at great levels of detail – under the new legislation for items on the Food Traceability List, said Herb.
Her advice for those preparing for the changes?
“First, I would look at that Food Traceability List and look at all the items on that list,” she said. “I would review my approved raw materials, and I would look at the Critical Tracking Events (CTEs) associated with those materials – and that would tell me which Key Data Elements that I need to capture. I would do the same thing with my finished goods and determine if I’m selling any finished goods. Am I going to do a transformation? Do I need to provide that information for shipping?”
Once you’ve collected all the relevant information, it’s advised you then start analyzing any gaps and begin the process of collecting that data from your suppliers.
“I’d probably be pulling together somebody in quality assurance, regulatory, purchasing, maybe IT, and saying ‘how are we going to get this?,” continued Herb. “Are you going to update our supplier survey and send it out now? Do you need to create any new fields in your computer system, or do you already have the fields you need? I’d look at the KDEs I need, which ones I already have, which ones I need to collect, how am I going to get that, and where am I going to put that information when I receive it?”
Software Simplifies the Process
Clearly, there will be a lot to manage – but digitizing records and utilizing software such as Datacor ERP can help businesses streamline their data collection and ensure they stay compliant with FSMA 204(d).
“Hopefully, you have an ERP or a robust computer system because I think managing all this data in spreadsheets or, heaven forbid, on paper would be very difficult or impossible,” warned Herb.
The webinar continued with a detailed breakdown of a sample report, with Herb and Finn talking through examples of the various KDEs that can be collected when goods are received, including:
- Bill of Lading Number
- Transporter Name
- Import Entry Number
- Traceability Lot Code
- Quantity and Unit of Measure
- Traceability Product Identifier
- Traceability Lot Code Generator Location Identifier
- Traceability Lot Code Generator Point of Contact Name
- Traceability Lot Code Generator Point of Contact Phone
- Traceability Lot Code Generator Email
- Location Identifier for where food we received
- Location Identifier for the immediate previous source
- Receipt Date and Time
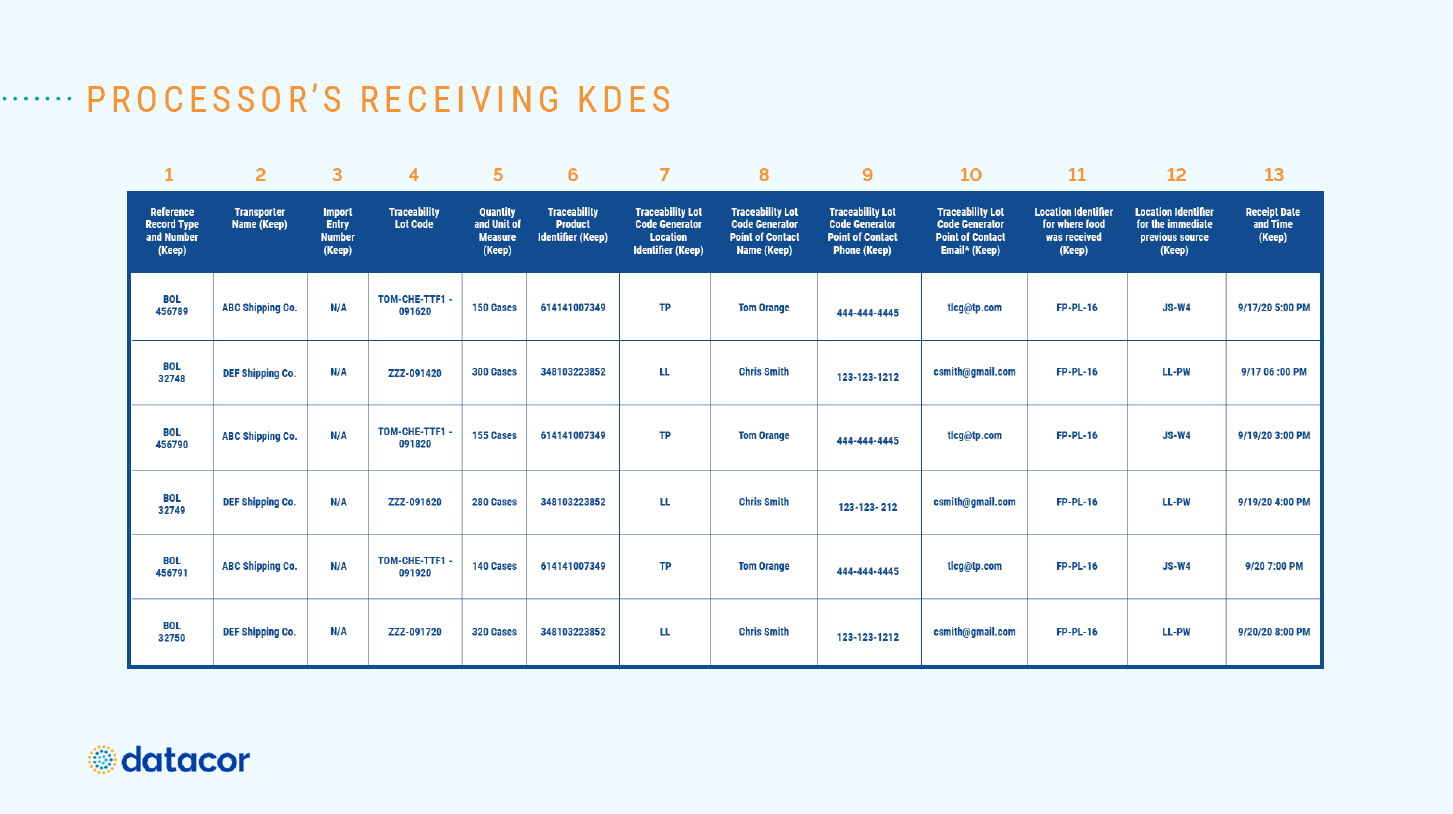
Herb noted that the FDA had gone to great lengths with this risk-based model to minimize the list of items and information required.
Finn agreed, “I really commend the FDA for the amount of documentation they’ve done. Lots of questions are answered in the documentation – but there’s so much. It is quite a bit to read through.”
Given these stricter requirements will be required in early 2026, auditors are now looking to educate everyone about the incoming changes, the Food Traceability list, and the KDEs for each stage of the tracking event.
Herb added, “By now, people really should be getting together with cross functional teams to address this because once you figure out all of the missing data, you need time to collect it. You need time to run some mock recalls, test it, and make sure it’s going to work.”
The discussion concluded with recommended steps food processors should be taking to gather all the required data and prepare for the new rules:
- Identify any food traceability list items that you receive or produce.
- Decide how to flag these items that are on the food traceability list in your database.
- Identify your role in the supply chain
- Ask what CTEs you’re involved with, so you know what your key data elements are.
- Determine whether there are any gaps in what is required and what you’re already collecting – and try to start filling in those missing pieces.
- Update your supplier surveys.
- Work with your IT department to include any additional fields, if necessary, and work with your technical teams to update the reports you generate. Ensure all the correct KDEs and columns appear, referring to the exact language and definitions required by the FDA.
- Determine what your company’s Traceability Lot Code source is.
- Implement a robust traceability plan. Even if you don’t have any items on the Food Traceability List, you will likely need a plan to show how you’re going to evaluate your raw materials.
- Lastly, conduct mock recalls. Select an item on the Food Traceability List and attempt to generate the reports that may be requested by the FDA within 24 hours.

Webinar highlights:
To watch the full webinar, visit https://datacorinc.wistia.com/medias/28g99eyq7m.
Below are some of the key takeaways from the session:
0:27 – Webinar overview
4:50 – The importance of food traceability
10:44 – Traceability of Lot Codes
17:30 – Being prepared for the changes
19:15 – Digitizing can help the process
20:00 – A deep dive into the KDEs
41:44 – FDA resources
48:25 – Q&A
52:15 – Getting ready

