
Multiple Basics of Food Product Health Claims are made on this product. Can you spot the difference?
Next to safety, content claims might be the most highly reviewed criteria of a food product by the FDA. During a time where functional foods have risen to exponential heights in consumer popularity, it is important to understand what types of claims there are and what parameters exist around making such a claim on product packaging. The Food and Drug Administration must uphold the highest standards regarding claims in order to ensure the health and safety of the public. As a result, they have gone to lengths to make sure that manufacturers have the proper resources to not only understand claims, but abide by them. As you very well know, the information set forth by the FDA can get a bit confusing, so we’re kicking of a 2 part article series to understand claim basics so that you can be sure to remain within the boundaries of safety when making claims on your food product packaging. You ready? Let’s begin.
Claim Types
There are 4 main types of content claims that the manufacturer must be aware of in order to ensure FDA compliance in product packaging. These claims speak to the nutrient content, health improving potential, and functionality of the ingredients contained within the food product or recipe.
Claim #1: Nutrient Content Claims
According to the FDA, a nutrient content claim speaks (directly or indirectly through implication) to the level of a nutrient contained within a food. These claims specifically refer to the actual nutrient content that is displayed on the nutrition facts panel. The categories that can describe nutrients are: free, low, or less/reduced. Great examples of a nutrient content claim are represented in (but not limited to) the following:
- Reduced Fat potato chips
- Sugar-Free syrup
- Low-fat yogurt
To make a nutrient content claim, the nutrient values within your product recipe must meet the specified criteria:
Free– Less than 5 cal per RACC and per labeled serving
Low – 40 cal or less per RACC (and per 50g if RA CC is small) OR Meals and main dishes: 120 calorles per 100 g
Less/Reduced – At least 25% fewer calories per RACC than an appropriate reference food (for meals and main dishes, at least 25% fewer calories per 100 g)
Lite/Light- 50% or more of the calories are from fat, fat must bereduced by at leas t 50% per RA CC. If less than 50% of calories are from fat, fat must be reduced at least 50%
or calories reduced at least 1/3 per RACC.
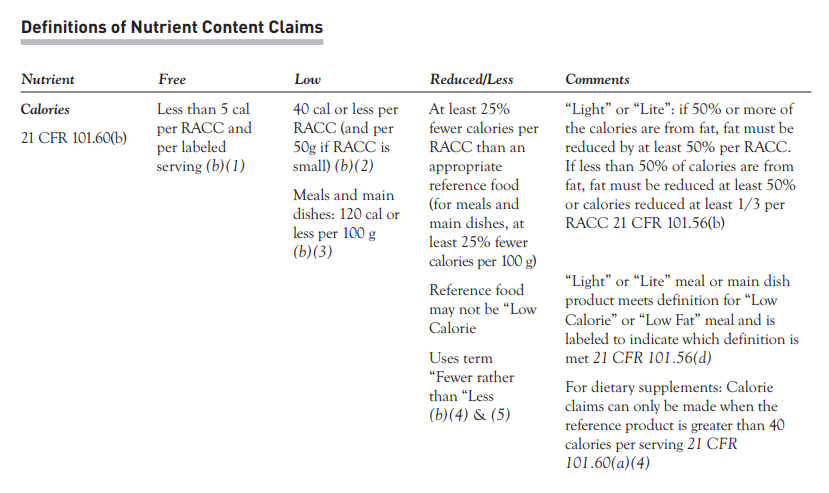
Here you can see the examples for making nutrient content claims around calories.
For additional information from the FDA on Nutrient Claims, Download this PDF and scroll to Appendix B.
Claim #2: Authorized Health Claims
An authorized health claim is a statement or symbol placed on product packaging that associates the product with a disease characterizes the relationship of any substance to a disease or health-related condition. Implied health claims include those statements, symbols, vignettes, or other forms of communication that suggest, within the context in which they are presented, that a relationship exists between the presence or level of a substance in a food and a disease or health-related condition. Authorized Health Claims must also be backed by substantial scientific evidence, meet the criteria outlined by the FDA to substantiate the claim and comply with the Scientific Scientific Agreement which denotes that there must be a substantial consensus among health and food science professionals regarding the proposed claim.
An example of an authorized health claim, is: “Three grams of soluble fiber from oatmeal daily in a diet low in saturated fat and cholesterol may reduce the risk of heart disease.”
But this claim must be substantiated by actual thresholds met according to FDA guidelines. In a hyper-simplistic example, to make the above claim, the oatmeal product mentioned must actually contain the soluble fiber that suits the claim. See FDA-provided chart for specific examples below.
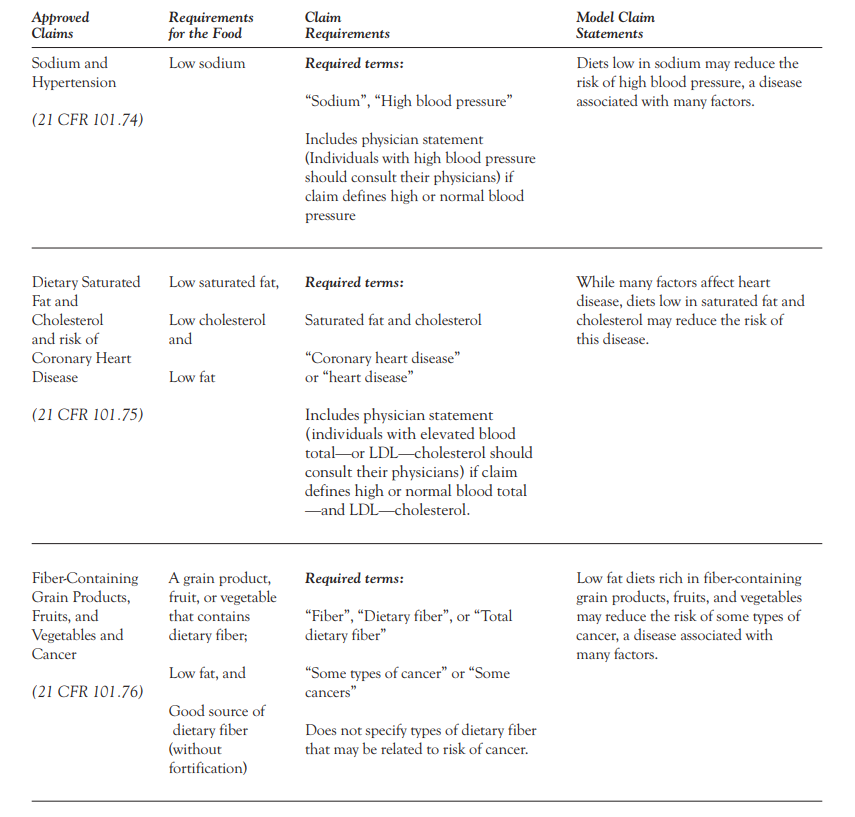
Here you can see a few examples of parameters published by the FDA for Health Claims.
For additional information from the FDA on Health Claims, Download this PDF and scroll to Appendix C.
Claim #3: Qualified Health Claims
Qualified food product health claims refer to a claim made on a food product label in reference to a disease that does not have to meet the rigorous standards of an Authorized Health Claim. A Qualified Health Claim must still be backed by substantial scientific evidence and must be presented with a qualifying statement such as: “Scientific evidence shows, but does not prove, that eating X amount of whole grains per day can reduce your risk for Type 2 Diabetes.”
Qualified health claims are a bit more flexible in nature and do not declare a direct outcome but leaves the information presented open to interpretation while being informative. Here’s an example of what the FDA expects to see in a Qualifying Health Claim:
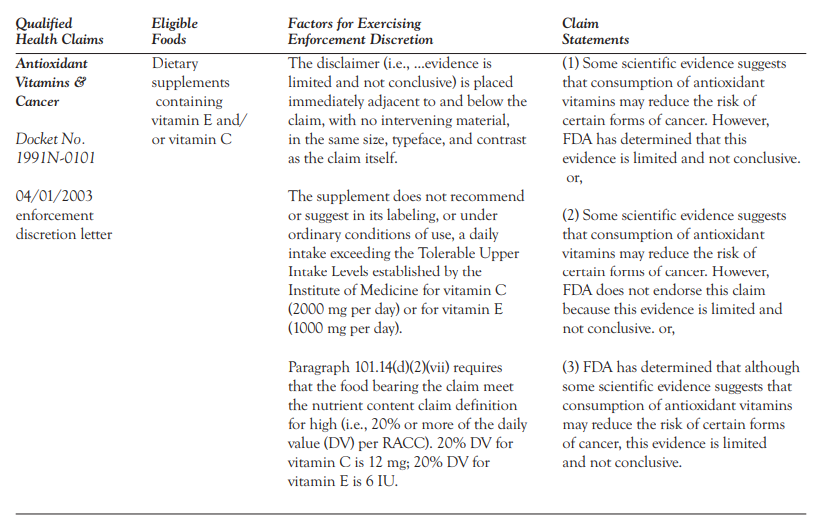
Claim #4: Structure/Functional Claims
Here’s where things can get a little bit confusing. Food product Health Claims and Structure/Function claims both talk about their impact upon health. However, the differentiating factor between the two is that Structure/Function claims refer to the nutrient or ingredient’s impact on overall health improvement and does not mention disease.
Health Claims are a highly reviewed area of product packaging due to the implications they make about disease improvement. These claims must be carefully made after substantial quantification in your nutritional reporting and we also recommend a thorough review by your legal team. Why? Messaging about the impact of a food on improving disease potential can be misrepresented and, if not regulated, can lead disease sufferers to believe that their disease can be improved and/or cured by the consumption of a food product under false pretenses. Simply put, it’s just plain false advertising.
On the other hand, structure/functional claims are not reviewed by the FDA and refer to and ingredient’s potential impact on an area of the body. A structural/functional claim example would be “calcium builds strong bones”. These claims are actually categorized alongside claims made on supplement labels. These claims must be truthful and not misleading but are not neither reviewed or backed by the FDA.
