FDA Food Labeling 101
The FDA does not pre-approve food labels. Good inventors, chefs, cooks, and others have given up on their dreams while waiting for approval for their labels. The Nutrition Labeling and Education Act of 1990 gave the FDA labeling authority. The Act mandates that the Food Labeling Areas of Interest determine their category.
The Food Labeling and Standards Department handles any questions about labels. Questions about warnings and safe handling should be presented to the FDA’s Warnings and Safe Handling Statements Department. Food labeling is one of the most frequently addressed issues of these departments. It can be very challenging, tedious, and seem unnecessary. But it is necessary to meet these requirements if you want to sell your product.
Basics for Food Labels to Meet FDA Requirements
Name of the product or statement of identity
Provide the name of your product. If your product gains popularity, you do not need to rename your company. Instead, check with your local Secretary of State office, do an online search, or pay for a business search to check the name. The US Patent and Trademark Office will not be helpful.
Net quantity of contents, or amount of product
This is the total quantity or amount of product in that packaging. For example, suppose your peanut butter cookies come in a package of six cookies. This section is where you would indicate that.
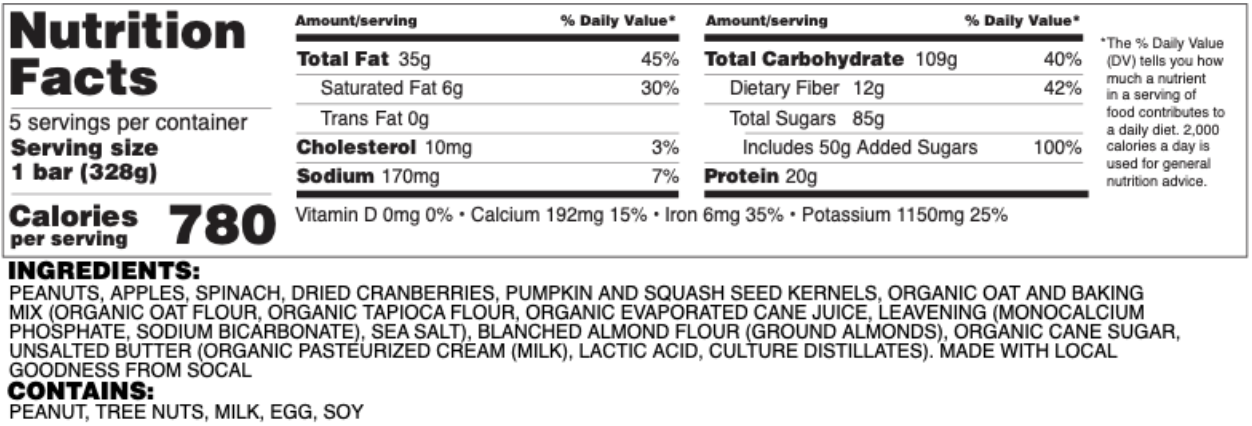
Nutrition Facts
- Ingredient List. List each ingredient in order of predominance in the recipe. List the flour first if you use one cup of sugar and two cups of flour. This list is not in the order of addition to the recipe.
- Name & Location of the Manufacturer, Packer, or Distributor. You can list the address of your home, packager, or distributor. If you are cooking, packaging, and shipping from home, list your home address. The food labels must have this information. A site visit by the FDA, USDA, and State Health Department could be necessary.
- Nutrition Information. This information details the serving size, total calories, cholesterol, fats, and sodium percentages. Also include total carbohydrates, sugar, and vitamins in this section. There are exemptions, rounding guidelines, and creative wording examples available in the FDA Food Labeling Guide.
- The Food Allergen Labeling and Consumer Protection Act Was Implemented in 2004. The FALCPA requires additional information if the recipe contains one of the major allergens. This information must be on the Information Panel. The FDA Food Labeling Guide has examples of several acceptable ways to do this.
Warnings and Safe Handling Statements
WARNING: This product has not been pasteurized and, therefore, may contain harmful bacteria that can cause serious illness in children, the elderly, and persons with weakened immune systems. (Code of Federal Regulation Chapter 21-Subchapter B)
The FDA requires this wording above if there are concerns about safe handling procedures.
28,000+ nutrition facts panels created. 1000’s of products brought successfully to retail.
Not a single recall.
Acceptable Location for Information on the Food Label
- The Principal Display Panel (PDP) is the portion of the label that the consumer sees first. There may also be alternate PDPs depending on the shape of the container. When there are alternate PDPs, select one as the PDP, or they must all have the required PDP information. The PDP identifies the product by name and the amount of product.
- The Information Panel is the panel directly to the right of the PDP. If this surface can not hold a label, it goes to the next panel to the right. This label includes the name and address of the manufacturer, packer, or distributor. In addition, the ingredient list, nutrition labeling, and any required allergy labeling also go here. Do not include any information other than this on The Information Panel.
