
New industry guidelines and food labeling requirements released August 2019 on the declaration of folate, niacin and vitamins A, D, & E on a food label.
New August 2019 FDA Industry Guidance for Food Labeling: Folate, Niacin, Vitamins A, D & E
As of August 2019, the FDA released new guidance for the industry concerning food labeling for certain micronutrients listed on the nutrition facts label on food products.
Folate, Niacin, as well as Vitamins A, D, E are all receiving a do-over in their appearance on the nutrition facts label to be more relevant to the terminology used by food scientists and equivalent experts when referring to their daily value as well as proper conversion for the new updated food label layout for 2019.
Industry Guidance vs. Requirements
As a quick note, the U.S. Food and Drug Administration releases industry guidance documents as a means of letting food manufacturers and other industry professionals know their thoughts on a subject. In this case, the appropriate conversion and presentation of particular nutrients and their daily values is the topic of concern. Often, industry guidance documents are released as a precursor to food labeling requirements and can be helpful for the food manufacturer to stay ahead of the curve in regards to upcoming changes.
Guidance Overview
According to the new food labeling guidance released by the U.S. Food and Drug Administration, the purpose of the document is to provide a step-by-step guide for conversion of the previous units of measure of folate, niacin, vitamins A, D and E. These conversions are to help assist food manufacturers in compliance with the food labeling requirements for the updated nutrition facts label format released earlier this year.
In 2016, the FDA released new updates for these nutrients and their daily values as seen in the chart below:
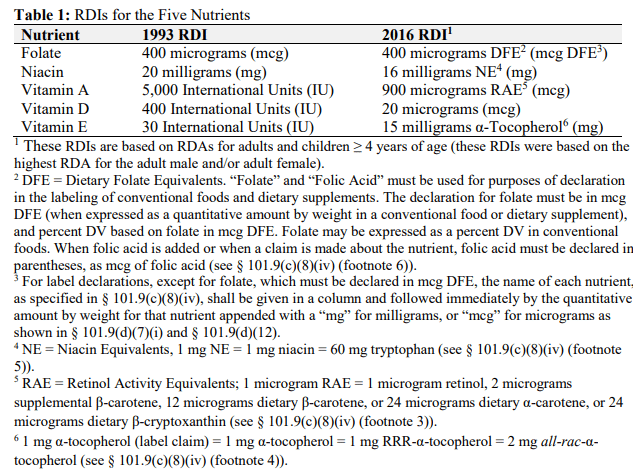
Updated food labeling requirements are mandatory for fat-soluble vitamins A, D & E
If you refer to the footnotes, they’re, well, noteworthy. The acronyms describing the units of measure of each nutrient are one of the biggest components changing in these updated conversions. Let’s go through these one by one.
Folate
The acronym DFE (Dietary Folate Equivalents) is used to describe the bioavailability of folate in it’s natural form as well as the synthetic form of the vitamin (folic acid) that is often used to fortify foods.
Quick note: Fortification is the term used when vitamins and minerals are added to a food that does not normally contain that nutrient or has the nutrient in small amounts. For example: Milk is often fortified with vitamin D because vitamin D facilitates the absorption of the calcium naturally found in milk).
According to the industry guideline released by the FDA:
“Because folic acid is 85 percent bioavailable, but naturally occurring folate is only about 50 percent bioavailable, folic acid is 1.7 (85 ÷ 50) times more bioavailable.
Therefore, the DFE folate is calculated as:
mcg DFE = mcg naturally occurring folate + (1.7 × mcg folic acid) “
In simple terms, to determine the % of DV (percentage of daily value) of folate in your product, all forms must be considered and combined if they exist in your recipe and then reflected on the nutrition facts label as pictured. See example below, taken directly from the FDA Industry Guideline:
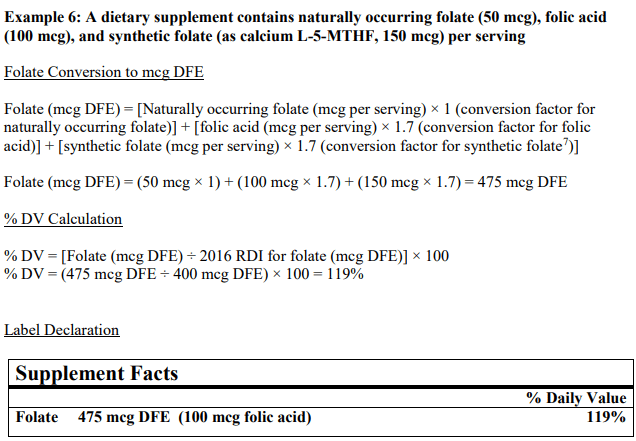
Updated folate/folic acid conversions consider the bioavailability and absorption of both naturally occurring and synthetic folate used for fortification.
Niacin
The expression of niacin in on the nutrition facts label on food products is reflected in the acronym NE, for niacin equivalents. According to www.fda.gov, the term niacin is descriptive of:
Below is an example of the conversion to reflect the daily value and NE of niacin in a food product that considers both niacin and tryptophan in it’s calculation:
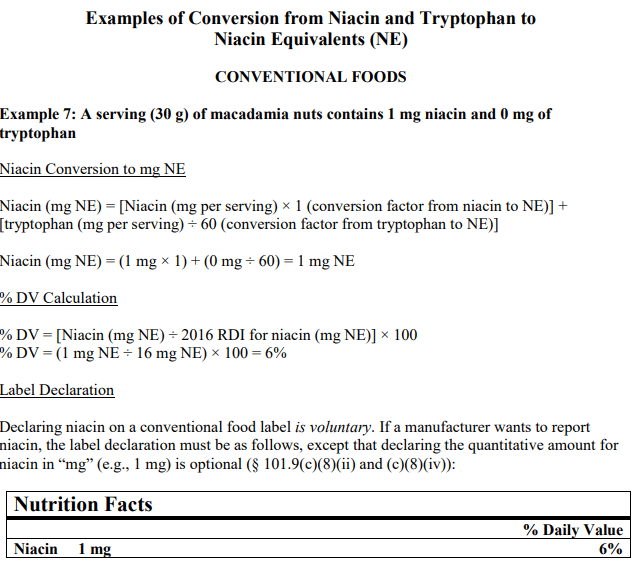
There are no current food labeling requirements stating mandatory declaration of Niacin on a food label, however, for customer information purposes, it is helpful to provide current nutrition information about your product.
Food companies have the option of listing niacin on a food label. It is voluntary and not considered a food labeling requirement, however, if the daily value of niacin is listed, this conversion is the most current and recommended to display the units of measure of niacin on food packaging. For more information on converting niacin for food labeling go to: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-converting-units-measure-folate-niacin-and-vitamins-d-and-e-nutrition-and
Vitamin A
In the past, Vitamin A has been previously expressed as IU (international units) on the nutrition facts label on food packaging. According to the food labeling guide for the updated conversions, that expression is no longer the most accurate because it does not calculate carotene (a form of vitamin A) correctly and convert it it RAE (retinol activity equivalents) for proper daily value percentages.
The updated conversion from IU’s to RAE can be seen in the chart below:
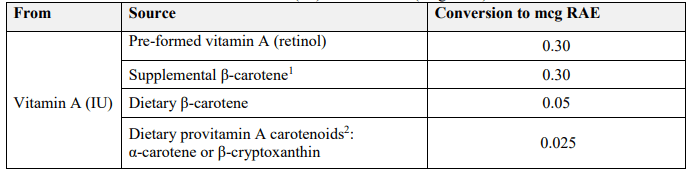
Per the FDA, food labeling requirements state mandatory declaration of Vitamin A on a food label. Conversion to RAE is the most updated formatting per the industry guideline.
Formula for Conversion from Carotenoids (mcg) and Pre-Formed Retinol (mcg) to Vitamin A (mcg RAE)
Vitamin A (mcg RAE) = [retinol (mcg per serving) x 1 (conversion factor for pre-formed retinol)] + [β-carotene (mcg per serving) ÷ 12 (conversion factor for β-carotene)] + [βcryptoxanthin (mcg per serving) ÷ 24 (conversion factor for β-cryptoxanthin)]
In an example where a food item contains 10 mcg of retinol, 1500 mcg of beta-carotene, and 1000 mcg of βcryptoxanthin the formula to calculate the combined mcg daily value would be as follows:
Vitamin A (mcg RAE) = (10 x 1) + (1500 ÷ 12) + (1000 ÷ 12)= 218.3 mcg RAE
From here to calculate the daily value percentage based on the recommended daily intake (RDI) for Vitamin A (which is 900 mcg RAE) the formula is as follows:
% DV = [Vitamin A (mcg RAE) ÷ 2016 RDI for vitamin A (mcg RAE)] × 100
So if we plug in our numbers from the example:
% DV = ( 218.3 mcg RAE ÷ 900 mcg RAE) × 100 = 24% (rounded down from 24.2)
So the daily value percentage based on proper conversion of our food item example would be approximately 24%. This number reflects what is appropriate for declaration on a food label considering the conversion from IU’s to mcg RAE. Declaration of Vitamin A on a label is voluntary, however, if you as a food manufacturer would like to declare Vitamin A on your label, this is the most appropriate calculation.
Vitamin D
Vitamin D, which is known to facilitate the absorption of calcium, is a fat-soluble vitamin which was also previously expressed in international units (IU’s) similar to Vitamin A. Not similar to our previous vitamin, vitamin D must be expressed on a label.
According to the new industry guideline, the best expression for Vitamin D is in mcg. This rule applies to all forms of vitamin D and their various combinations with one another.
40 IU= 1 mcg of Vitamin D
To convert Vitamin D from IU’s to mcg, the formula is as follows:
Vitamin D (mcg) = Vitamin D (IU per serving) × 0.025 (conversion factor for vitamin D)
For example, if a food item contained 100 IU’s of Vitamin D, the conversion would look like this:
Vitamin D (mcg) = 100 IU × 0.025 = 2.5 mcg
Then if we wanted to calculate the daily value percentage for that particular food item the formula would look like this:
% DV = [Vitamin D (mcg) ÷ 2016 RDI for vitamin D (mcg)] × 100
Plugging in our numbers would result in approximately a 13% daily value percentage of Vitamin D expressed in mcg for correct food label formatting:
% DV = (2.5 mcg ÷ 20 mcg) × 100 = 13%
Vitamin E
As I’m sure you can guess, Vitamin E and it’s various forms were also previously expressed in international units on a food label. The industry guide recommends that this vitamin also be converted to mcg for accurate expression.
Below is an example including all conversions directly from the FDA Industry Guide:
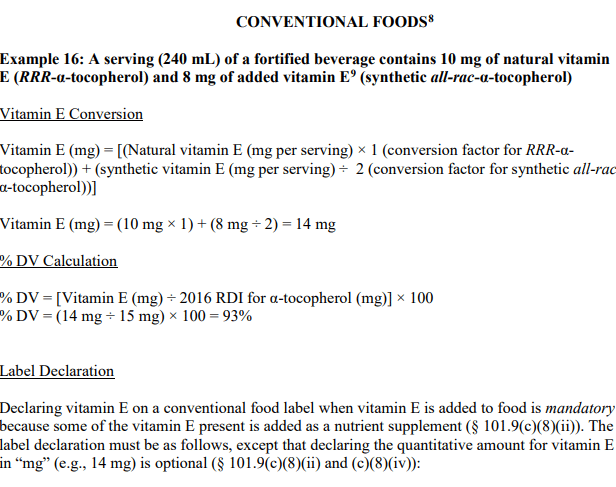
As mentioned in the label declaration portion of the snippet above, Vitamin is must be declared on a label when it is added to a food not normally containing this nutrient (likely through fortification).
Decoding these Conversions
Industry guides released by the FDA contain the most up-to-date thoughts of the U.S. Food and Drug Administration regarding food manufacturing, nutrition, food labeling and other aspects of the food industry. When attempting to accurately express micronutrients on a label according to FDA industry standards, it is helpful to utilize software and even a licensed professional to ensure accuracy and proper format of your label declarations.
LabelCalc is an industry leading nutrition analysis software, complete with expert consulting from a Registered Dietitian upon request. If you have questions or concerns regarding your food label and compliance with the most recent standards of the FDA, contact us today by visiting www.labelcalc.com.

